PO/3P® SMART culture media
EM-BRACE TRACEABILITY
ACCURACY IS EVERYTHING.
Accuracy is essential when detecting contamination, it helps empower you to make smarter decisions, and to keep your products, employees, and consumers safe so your business stays compliant. So, when it comes to environmental monitoring, it is absolutely crucial to equip your site with state-of-the-art culture media — specifically designed for more definitive results, and has been re-engineered to trace itself.
The 3P® legacy is one of delivering proven performance when it comes to effective pharmaceutical contamination control. Now it’s going digital.
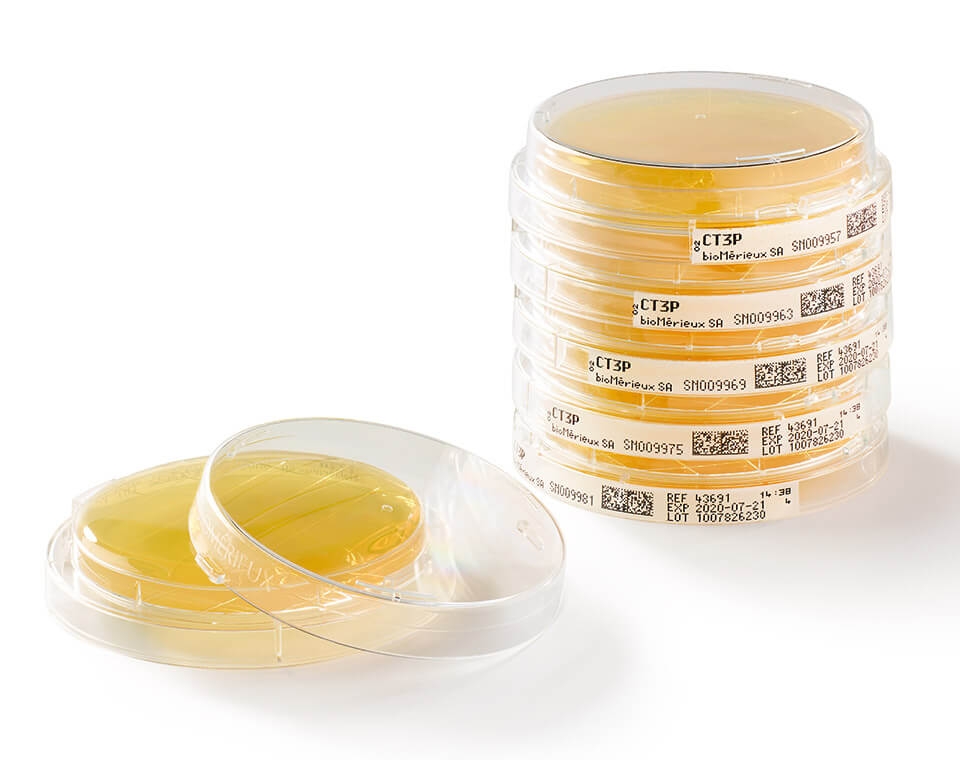
With GS1 barcodes and LOCKSURE® lids that decrease risk of manual errors or accidents and bioMérieux’s new ultra-clear design that makes identifying and counting colonies easier through the lid, we can help keep you compliant and audit ready.
DATA INTEGRITY GETS AN UPGRADE.
● Accurately manage the contamination level of protected areas thanks to 3P® reliable performances and optimized neutralization through the entire shelf-life.
● Confidently secure the handling and transport of your EM samples with LOCKSURE® lids.
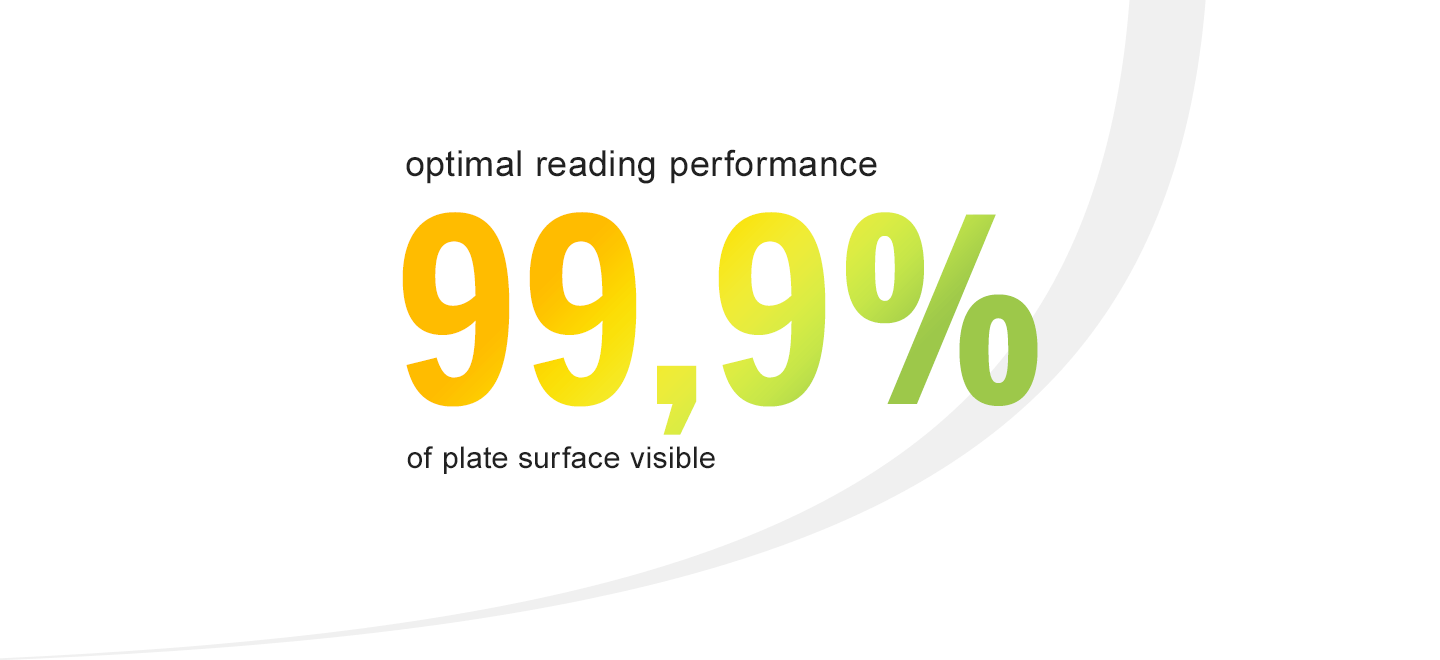
● Optimize visual inspection of your EM samples with clear plate design allowing 99,9% of the visible surface plate area for more accurate counting results.
● Instantaneously and consistently ensure traceability & data accuracy with the gold standard GS1 barcode.
● Entirely digitalized EM to streamline process and reduce human error through 3P® CONNECT and 3P® ENTERPRISE.
A NEW SYSTEM IS EM-ERGING
Increase digitalization of your entire EM process through 3P CONNECT®
➔ See the video
To meet your expectations as well as possible, we are also developing a set of services, from validation to delivery embedded in 3P® Smart Plate offer:
Validation ready: validation studies, delivery free batches, double sourcing.
Ordering and delivery process efficiency: order consolidation and customer order tracking.
QC & stock management optimization: large monolots, RSL > 12 weeks, high level of OTIF.
➔ Discover our Supply chain Services and our complete Services ecosystem
➔ Visit the dedicated 3P environmental monitoring website


Conformidade com as regulamentações

Capacidade de neutralização confirmada contra 10 desinfetantes comumente utilizados nas salas limpas

Prazo de validade estendido, garantindo desempenho excelente e consistente até o último dia
Condições de armazenagem flexíveis entre 2-25°C
Teste de Promoção do Crescimento realizado com mais de 100 micro-organismos, incluindo leveduras, fungos filamentosos, bacilos, cocos, bactérias anaeróbias e Isolados Selvagens ("in house")

Compativel com isoladores com descontaminação VHP e PA

Monolote com lotes grandes

Transporte e uso seguros com o nosso exclusivo sistema LOCKSURE®

99,9% of the plate surface visible through the lid for optimized reading performance

GS1 barcode allowing to easily identify and track both samples and culture media

3P® range represents state-of-the-art culture media technology from both a formulation and packaging perspective.
For a fully integrated environmental monitoring solution and a reliable standard of quality, bioMérieux provides a global range of products benefiting from over 50 years of microbiology expertise.
GLOVE & AIR SAMPLING
90mm plates
- TSA 3P irradiated
- TSA 3P with Neutralizers irradiated
- Sabouraud 3P irradiated
SURFACE SAMPLING
55 mm plate
- Count-Tact 3P irradiated
- Count-Tact 3P + B lactamase irradiated
- Count-Tact 3P Sabouraud irradiated
- Count-Tact 3P with Enhanced Neutralizers irradiated
INSTRUMENTS & ACCESSORIES
- AIR IDEAL® 3P®
- BI-BOX irradiated
- QUANTISWAB® irradiated
- COUNT-TACT® Applicator