Bacillus subtilis: How it affects the Pharma Industry?
What is Bacillus Subtilis
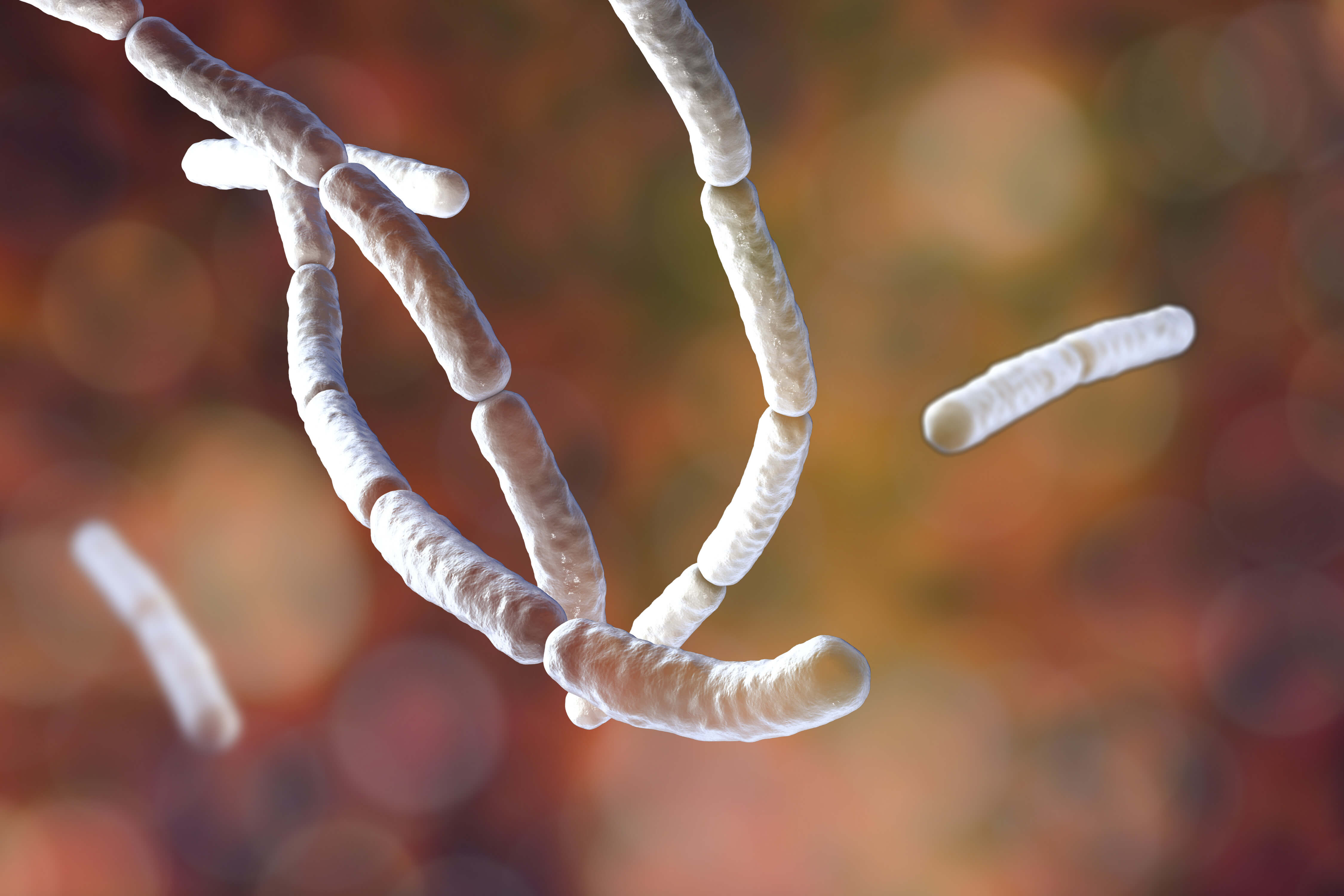
Bacillus subtilis is a gram-positive bacteria found in soil and the gastrointestinal tract of ruminants and humans. It is rod-shaped and can form a tough, protective endospore, allowing it to tolerate extreme environmental conditions1. Bacillus subtilis is considered the best studied gram-positive bacterium and a model organism to investigate bacterial chromosome replication and cell differentiation. It is also one of the bacterial champions in secreted enzyme production and is used on an industrial scale by biotechnology companies.
This article gives an overview of :
● The transfer of Bacillus subtilis
● The pathogen detection, prevention and control of Bacillus subtilis
● The risks of Bacillus subtilis for consumers
● Treating Bacillus subtilis
● Overview of industries affected by Bacillus subtilis
● bioMérieux’s products and solutions for detecting and preventing Bacillus subtilis
Prevention, Detection and Treatment
The Bacillus subtilis microorganism is ubiquitous in many environments. It is considered as an opportunistic pathogen, meaning the organism only causes disease when a person's immune system is already impaired. Bacillus subtilis is a common source of environmental contamination in the laboratory and is routinely found on environmental settle plates and in active air monitoring.
How can Bacillus subtilis be prevented and controlled in the pharmaceutical industry?
The Bacillus subtilis pathogen can be prevented and controlled in drug manufacturing by setting up cleaning practices in target risk areas that involve the use of chemical agents for removal of soil and for control of microorganisms. Disinfectants, sanitizers, sporicidal chemicals or detergents can be used, and a robust cleaning program for non-product contact surfaces, in line with industry control guidelines, should be developed involving the relevant combination of chemical products.
How can the presence of Bacillus subtilis be detected and controlled in the pharmaceutical industry?
Bacillus subtilis can be detected using media such as Soybean-casein Digest Agar or Soybean – casein digest Broth 30°C - 35°C. As appropriate, additional laboratory tests may be needed to determine if products are suitable for release. Analysts should ensure that the methods used to test finished drug products prior to release for distribution are appropriately validated, accurate, sensitive, specific and reproducible.
What are the risks of Bacillus subtilis to the consumer?
Healthy people are usually at low risk of infection from Bacillus subtilis unless otherwise immunocompromised.
Bacillus subtilis produces an extracellular toxin known as subtilisin which, although having very low toxigenic properties, is capable of causing allergic reactions in individuals who are repeatedly exposed to it. Sensitivity of workers to subtilisin can be a problem in fermentation facilities where exposure to high concentrations occurs.
What is the treatment for Bacillus subtilis contamination?
The pathogenic potential of Bacillus subtilis is very low, when it exists at all2. Only a very small number of cases of infections due to Bacillus subtilis have been reported and each time antibiotic therapy was deployed as treatment.
Key Figures
-
Bacillus subtilis is heavily flagellated, which gives it the ability to move quickly in liquids.
It is one of the bacterial champions in secreted enzyme production and used on an industrial scale by biotechnology companies.
B. subtilis endospores serve as one of the models for evaluating the effectiveness of sporicides and sterilants.
When stressed, Bacillus subtilis transforms itself into a spore and enters a dormant state, allowing it to tolerate extreme environmental conditions.
Bacillus subtilis holds the record for surviving in space for the longest duration, 6 years on a NASA satellite.What common industries are affected by Bacillus subtilis?
Drug manufacturers of non-sterile products
Medical device systems
Cell therapy manufacturers.